Lorraine M. Albritton, PhD

Professor, Department of Microbiology, Immunology and Biochemistry
Professor, Department of Medical Education
601F Molecular Science Building
858 Madison Avenue
Memphis, TN 38163
Email: lalbritton@uthsc.edu
Phone: 901.448.5521
Fax: 901.448.7360
Education
- Postdoctoral Fellowship, Brigham & Women’s Hospital, Harvard Medical School, 1986-1991
- PhD, University of Tennessee-Oak Ridge National Laboratories Graduate School, Biomedical Sciences, 1986
- BS, Northeast Louisiana University, Physics, 1972
Research Interests
Two major interests drive my research programs – mechanisms of retroviral entry and development of retroviral and lentiviral vectors for gene therapy of cancer and human diseases.
A major focus has been to develop a strategy for targeting viral vector gene delivery to a specific cell type by modifying the viral envelope protein to use a cell type-specific membrane protein as its entry receptor. In previous years, we identified a fourteen amino acid receptor binding sequence (RBS) in the envelope protein of the murine ecotropic retroviruses (ENV). We then reduced to practice a novel concept for attaining targeted entry: replacement of the RBS with the sequences of a peptide ligand known to bind a cell-specific receptor expressed on the desired target cell population. The prototype construct contains the sequence of the peptide hormone somatostatin replacing the RBS and retroviral vectors coated with this modified Env infect cells expressing human somatostatin receptors at titers of over 106 transducing units/ml, levels comparable to infection of the wildtype virus using its natural retroviral receptor. In vivo studies in mice demonstrated infection of somatostatin receptor-positive neurons identified by expression of NeuN in the cortex, striatum and subthalamic nucleus by mKate-transducing lentiviral vectors containing the somatostatin receptor-targeted Env. The in vivo infection was specific to cells expressing somatostatin receptors (SSTR), as judged by co-immunostaining of cells with anti-SSTR antibody and reductions in infection when target receptors were bound by somatostatin peptide immediately prior to viral vector injection.
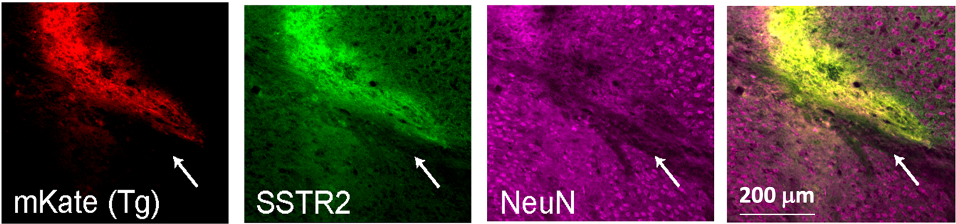
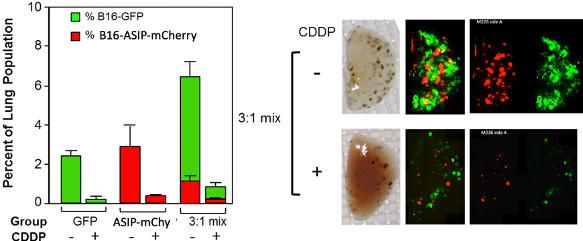
In collaboration with Dr. Michael Whitt, further modifications of the targeting ENV created a Somatostatin Targeting Proteins (STP) that incorporates into the oncolytic virus Vesicular Stomatitis Virus (VSV) in place of the normal VSV glycoprotein and gives entry-restricted infection of somatostatin receptor-positive cells. We developed an anti-melanoma transgene, agouti signaling protein (ASIP), a natural antagonist of MCR1 (melanocortin receptor 1), a major growth factor receptor for melanoma cells and determinant of tumor cell multidrug resistance. Delivery of a constitutively expressed ASIP gene to mouse melanoma B16-F10 cells significantly reduced the number and size of tumors in a syngeneic mouse lung model of melanoma metastases. In addition, ASIP gene delivery resulted in increased killing of melanoma cells by the chemotherapy agents cisplatin (CDDP) and gemcitabine. In vivo treatment using cisplatin almost completely cleared the lungs of tumors expressing ASIP but had little effect in mice with tumors that did not express ASIP constitutively.
In our most recent studies, we developed a set of three retroviral and lentiviral
vectors that deliver an anti-CD19 chimeric antigen receptor (CAR) to primary mouse
T cells. In collaboration with immunologists Dr. Marko Radic and Dr. Tony Marion,
the goal was to determine if, when implanted into mice models of spontaneous autoimmune
Systemic Lupus Erythematosus, anti-CD19 CAR engineered T cells would deplete the B
cell population and thereby halt production of pathogenic antibodies. We also sought
to determine if B cell depletion and pathogenic antibody reduction could be of therapeutic
benefit. We showed that while all three vectors delivered the CAR gene to comparable
percentages of T cells, they differed in the ability to deplete B cells in recipient
mice. Retroviral vectors coated with amphotropic retrovirus ENV was superior to retroviral
and lentiviral vectors coated with G protein from VSV, suggesting that the entry receptor
influenced the therapeutic properties of anti-CD19 CAR-engineered T cells, perhaps
by focusing gene delivery to a subset of primary T cells with higher capacity for
CAR expression and function or with higher capacity for engraftment in mice. Lupus
mice receiving T cells engineered to express the anti-CD19 CAR showed B cell depletion,
reduction of immune complexes in their kidneys and anti-nuclear antibodies in their
blood, amelioration of skin lesions and most lived a span comparable to the normal
laboratory mouse lifespan. Reversal of disease was due to persisting anti-CD19-CAR
T cells as judged by the ability of adoptively transferred T cells to reverse disease
in naïve Lupus mice even 10 months after CAR T cell administration.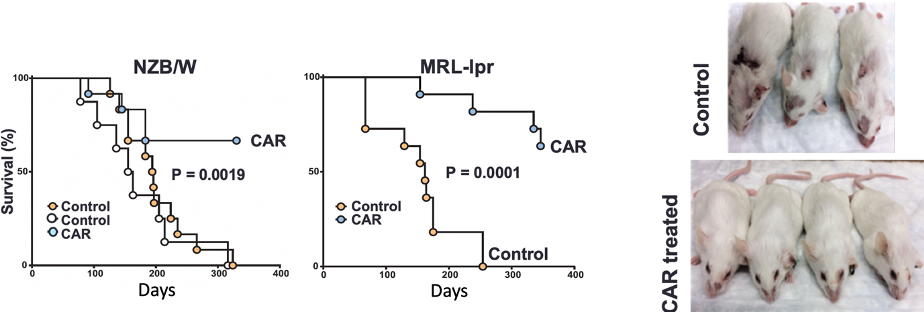
Scholarly Interests
Dr. Albritton served on the Recombinant DNA Advisory Committee (RAC) to the Director of NIH from 2016-2018 and was appointed by the Director to serve on his Novel and Exceptional Technologies Advisory Committee (NExTRAC) when it was organized from the former RAC in 2018. Currently, the committee is working on development of a framework under which it will function in the future and is charged with assisting the Director and the NIH Office of Science Policy in the development of a path forward for biomedical research involving gene drive-modified organisms for biomedical research. Dr. Albritton serves on the Working Group to Establish a NExTRAC Framework to assess potential issues that may occur as new biotechnologies are developed and applied.
